In The Following Experiment A Coffee Cup Calorimeter Containing 100
In the following experiment a coffee cup calorimeter containing 100 g of h 2 o is used. The initial temperature of the calorimeter is 230 o c.
in the following experiment a coffee cup calorimeter containing 100
in the following experiment a coffee cup calorimeter containing 100 is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in in the following experiment a coffee cup calorimeter containing 100 content depends on the source site. We hope you do not use it for commercial purposes.
The initial temperature of the calorimeter is 230 c.
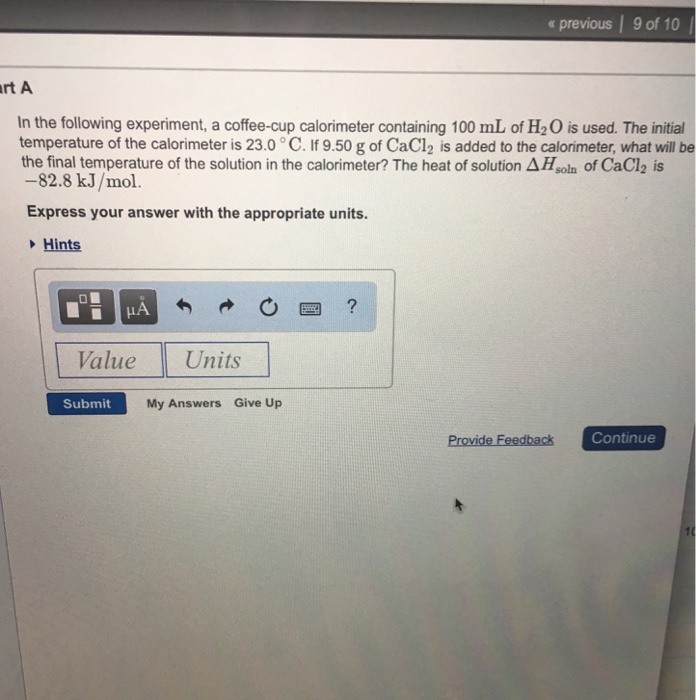
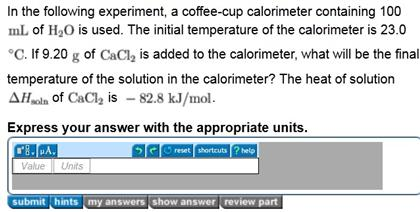
In the following experiment a coffee cup calorimeter containing 100. The initial temperature of the calorimeter is 230 circ c. If 970 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. The heat of the solution is delta h soln of cacl2 is 828 kjmol.
The initial temperature of the calorimeter is 230 degrees c. Express your answer with the appropriate units. In the following experiment a coffee cup calorimeter containing 100.
Chemistry qa library in the following experiment a coffee cup calorimeter containing 100 ml of h2o is used. If 47 g of cacl 2 is added to the calorimeter what will be the final temperature of the solution in the. In the following experiment a coffee cup calorimeter containing 100 g of h2o is used.
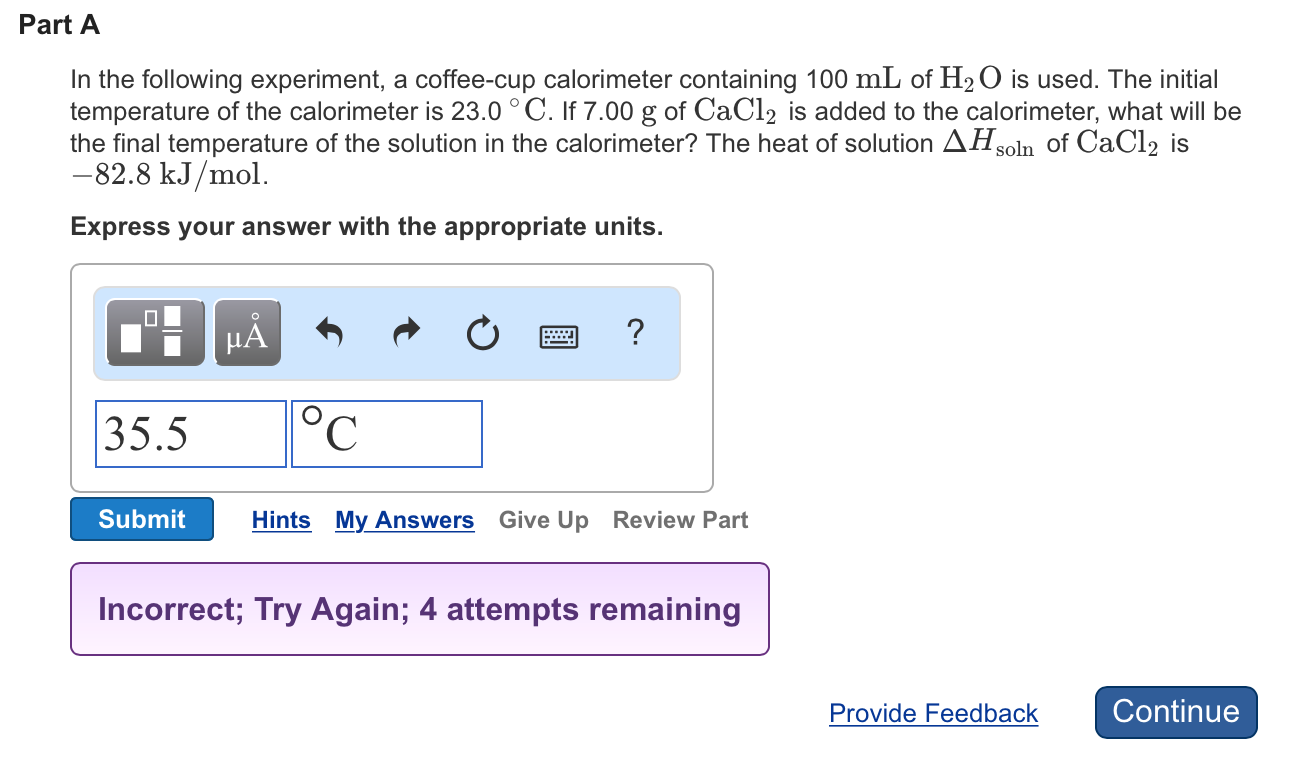
In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used. If 800 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. Ml of h2o is used.
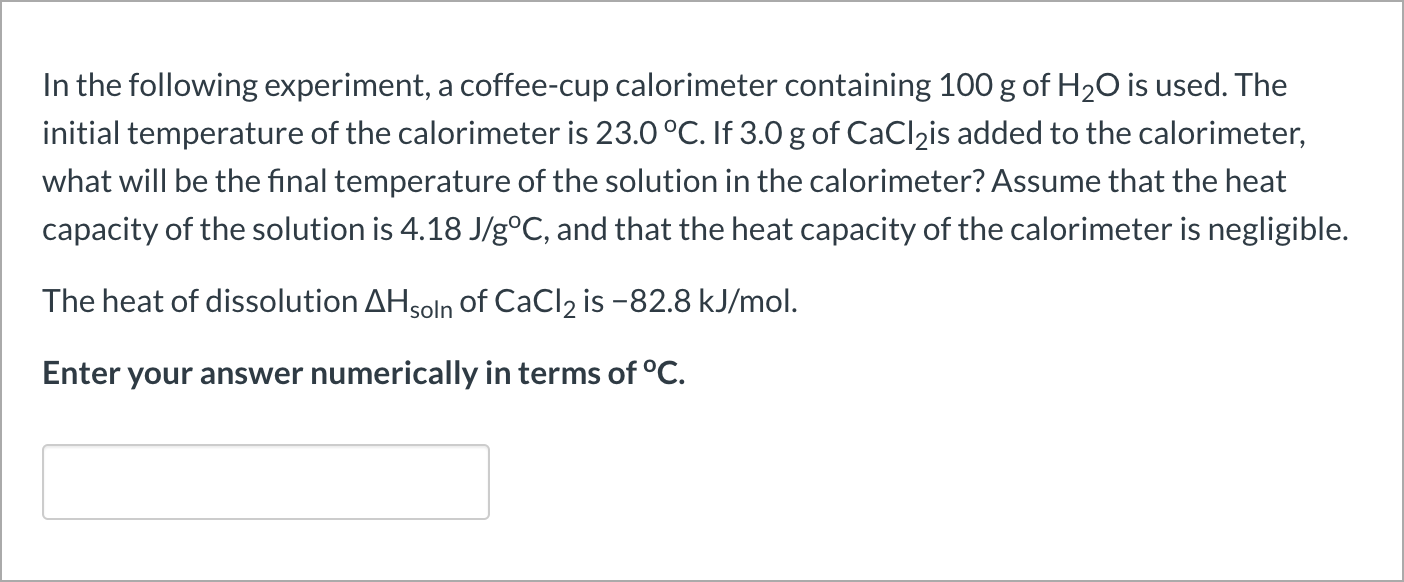
In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used. The heat of solution quoln of cacl2 is 828 kj mol1. If 550 g of cacl is added to the calorimeter what will be the final temperature of the solution in the calorimeter.
The heat of solution dhsoln of cacl2 is 828 kjmol. If 67 g of cacl2 is added to the calorimeter. The initial temperature of the calorimeter is 230 0c.
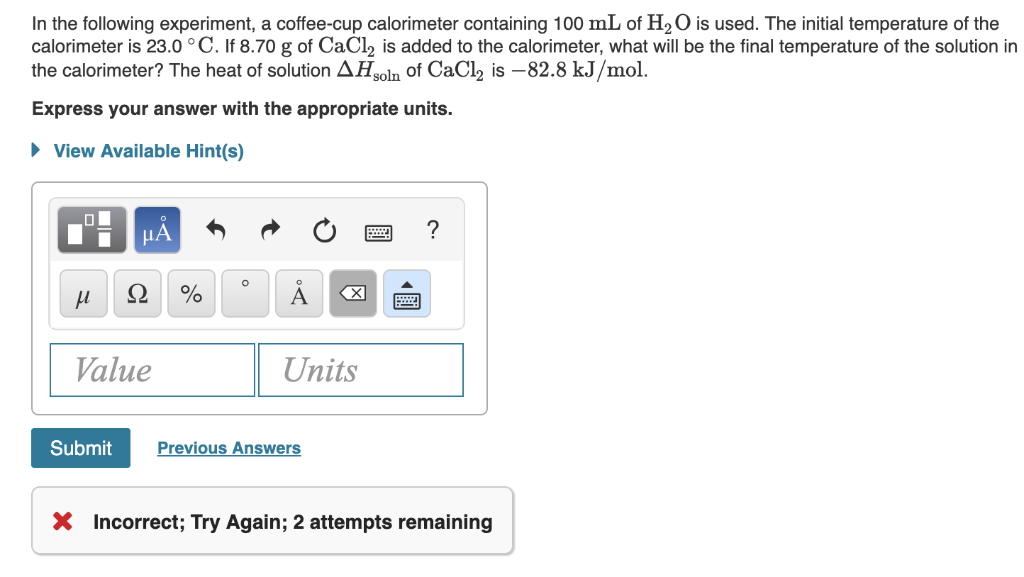
The initial temperature of the calorimeter is 230 c. If 840g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter.
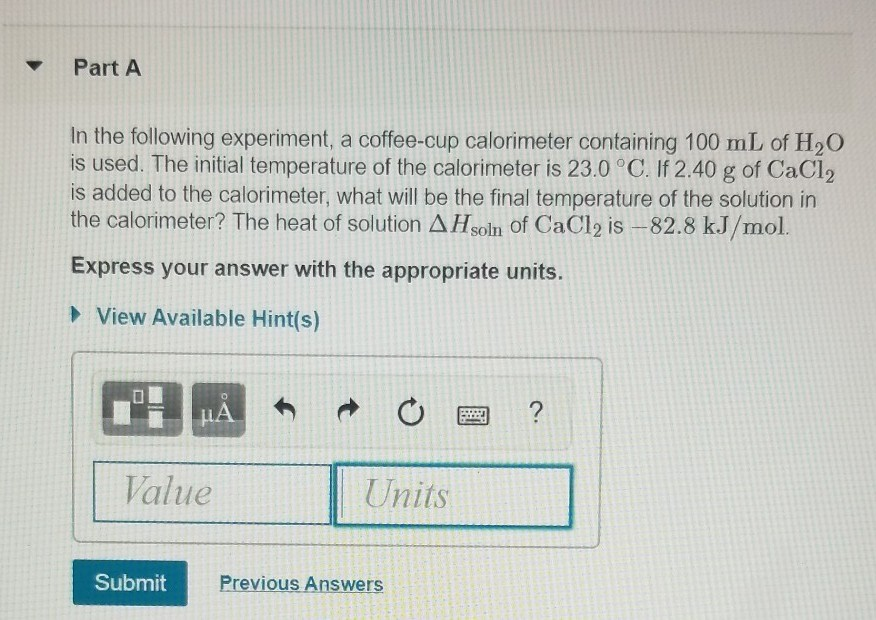 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
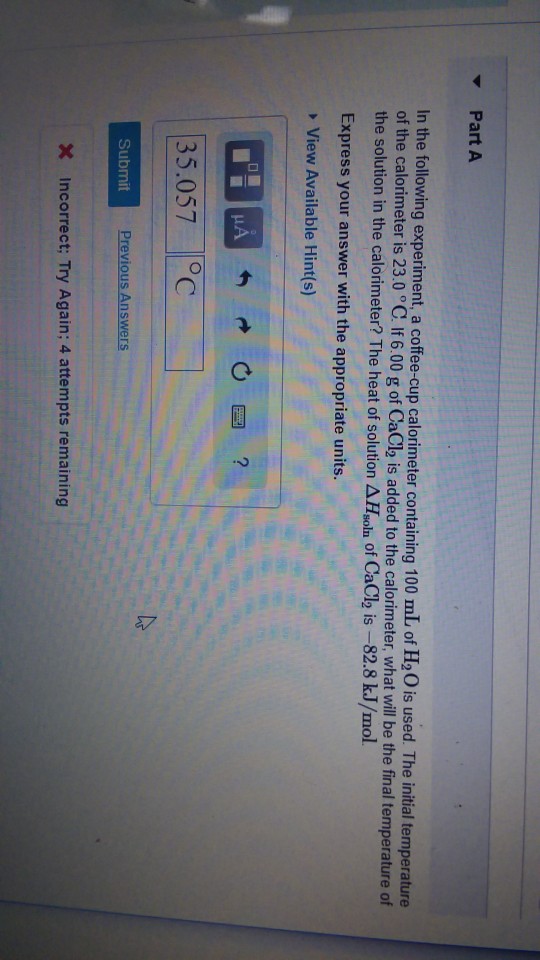
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
 In The Following Experiment A Coffee Cup Calorime Chegg Com
In The Following Experiment A Coffee Cup Calorime Chegg Com
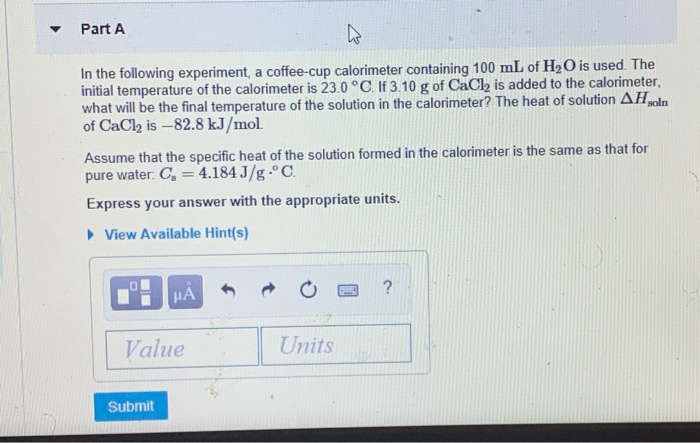 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
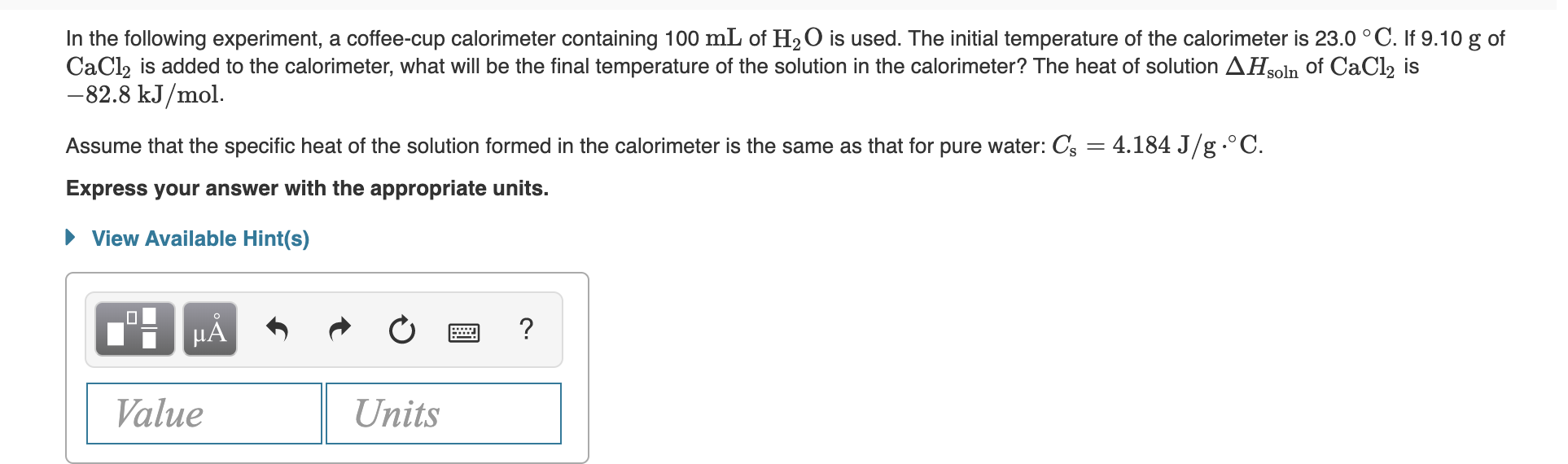 Answered In The Following Experiment A Bartleby
Answered In The Following Experiment A Bartleby
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
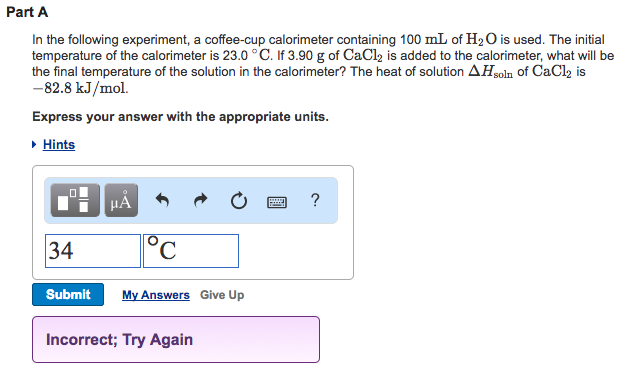 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
 Oneclass In The Following Experiment A Coffee Cup Calorimeter Containing 100 Ml Of H2o Is Used The
Oneclass In The Following Experiment A Coffee Cup Calorimeter Containing 100 Ml Of H2o Is Used The