In A Coffee Cup Calorimeter 1000 G Of H2o
After the reaction the temperature of both substances is 313 c. In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hci are mixed.
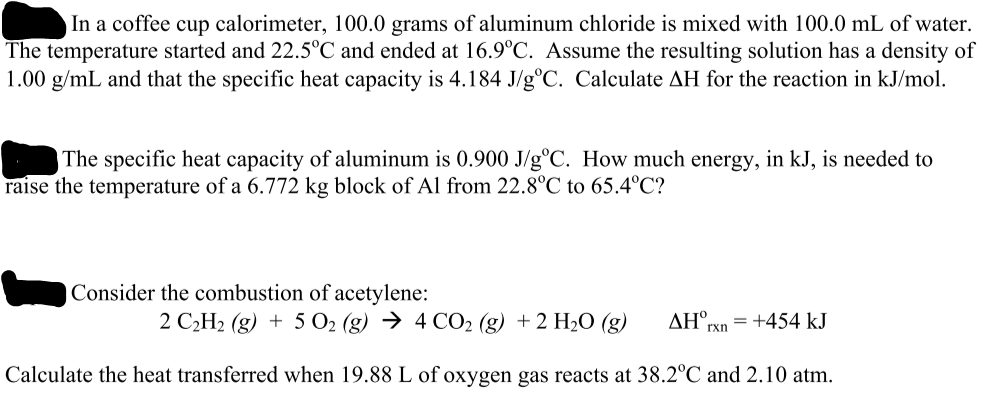 Solved In A Coffee Cup Calorimeter 100 0 Grams Of Alumin Chegg Com
Solved In A Coffee Cup Calorimeter 100 0 Grams Of Alumin Chegg Com
in a coffee cup calorimeter 1000 g of h2o
in a coffee cup calorimeter 1000 g of h2o is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in in a coffee cup calorimeter 1000 g of h2o content depends on the source site. We hope you do not use it for commercial purposes.
After the reaction the final temperature is 3130c.
In a coffee cup calorimeter 1000 g of h2o. In a coffee cup calorimeter 1000 g of h 2 o and 1000 ml of hcl are mixed. The hydro chloric acid hcl had an initial temperature of 446 0c and the water was originally at 2460c. Give your answer in units of joules per gram of compound.
In the following experiment a coffee cup calorimeter containing 100 g of h2o is used. In a coffee cup calorimeter experiment 1000 g of a soluble ionic compound was added to the calorimeter containing 750 g h2o initially at 2320c. The initial temperature of the calorimeter is.
The temperature of the calorimeter contents drops from 230 to 130. Assuming the specific heat of the solution and products is 420 jg 0c calculate the approximate amount of heat in joules produced. We consider the density of the solutions to be the same as water 100 gml both before and after mixing and note the temperature changes from 2500 deg c to 3200 deg c.
In a coffee cup calorimeter 300 g of nh4cl is dissolved into 900 g of h2o. Assume that the specific heat of the solution is the same as that of. View unit6test from chem ap at chapin high.
The final temperature of the solution was 3180c. Assuming that all the solutions have a density of 10 gcm 3 and a specific heat capacity of 418 j0cg calculate the enthalpy change for the neutralization of hcl by naoh. When 500 g of 0200 m naclaq at 241 0c is added to 1000 g of 0100 m agno 3 aq at 241 0c in a calorimeter the temperature increases to 252 0c as agcls forms.
The two solutions were initially at 22600c and the final temperature is 23400c calculate the heat that accompanies this reaction in kjmol of agcl formed. Both solutions were originally at 246oc. We pour into a an ideal calorimeter 1000 ml of 100 m sulfuric acid with 1000 ml of 100 m naoh all at 2500 deg c.
In a coffee cup calorimeter 1000 g of water h2o and 1000 ml of hydro chloric acid hcl are mixed. What was the change in enthalpy for the dissolution of this compound. The hcl had an initial temperature of 446oc and the water was originally at 246oc.
In a coffee cup calorimeter 500 ml of 0100 m agno 3 and 500 ml of 0100 m hcl are mixed to yield the following reaction.
 Solved 6 In A Coffee Cup Calorimeter 100 0 G Of H20 And Chegg Com
Solved 6 In A Coffee Cup Calorimeter 100 0 G Of H20 And Chegg Com
 Solved 7 A Coffee Cup Calorimeter Contains 100 0 G Of Wa Chegg Com
Solved 7 A Coffee Cup Calorimeter Contains 100 0 G Of Wa Chegg Com
 Ap Chemistry Ch 6 Thermochemistry Energy Is The Ability To Do Work Or Produce Heat The Sum Of All Of The Potential And Kinetic Energy Ppt Download
Ap Chemistry Ch 6 Thermochemistry Energy Is The Ability To Do Work Or Produce Heat The Sum Of All Of The Potential And Kinetic Energy Ppt Download

 Answered A Piece Of Metal Weighing 59 0 G Was Bartleby
Answered A Piece Of Metal Weighing 59 0 G Was Bartleby
 Solved Calorimetry Homework S H Of Water 4 18g J C 1 Wh Chegg Com
Solved Calorimetry Homework S H Of Water 4 18g J C 1 Wh Chegg Com

 Oneclass A 100 0ml Solution Of 1 05 H2so4 At 23 2degrees Celcius Is Mixed With 100 0ml Of 2 0 M Naoh
Oneclass A 100 0ml Solution Of 1 05 H2so4 At 23 2degrees Celcius Is Mixed With 100 0ml Of 2 0 M Naoh
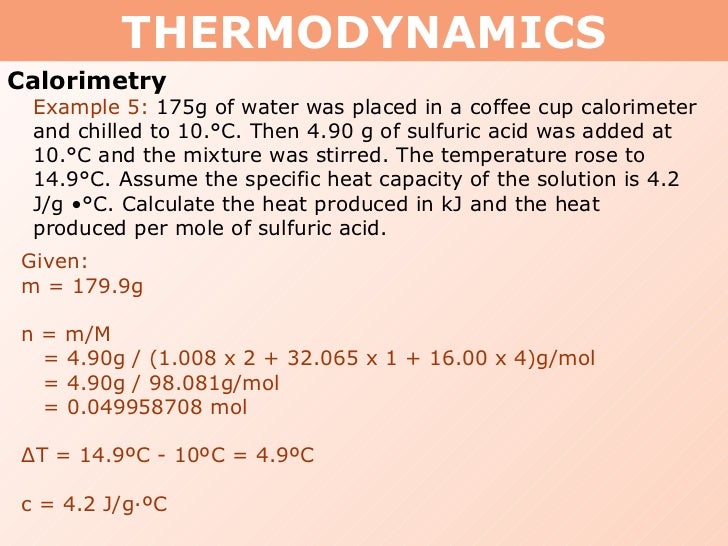 Tang 01 Heat Capacity And Calorimetry
Tang 01 Heat Capacity And Calorimetry