In A Coffee Cup Calorimeter 1000 Ml Of 10 M Naoh
Both solutions were originally at 246oc. After the reaction the nal temperature is 3138c.

in a coffee cup calorimeter 1000 ml of 10 m naoh
in a coffee cup calorimeter 1000 ml of 10 m naoh is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in in a coffee cup calorimeter 1000 ml of 10 m naoh content depends on the source site. We hope you do not use it for commercial purposes.
In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hci are mixed.

In a coffee cup calorimeter 1000 ml of 10 m naoh. Chemistry chemistry in a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hci are mixed. Agaq cl aq agcls the two solutions were initially at 22550c and the final temperature is 23710c. Both solutions were originally at 246c.
Both solutions were originally at 2468c. Both solutions were originally at 2450c. Assume that the combined solution has a mass of 1000 g and has a specific heat.
In a coffee cup calorimeter 1000 mathrmml of 10 mathrmm naoh and 1000 mathrmml of 10 mathrmm mathrmhcl are mixed. In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hcl are mixed. After the reaction the final temperature is 313c.
Both solutions were orignially at 2460c. Both solutions were origi nally at 2468c. Assuming that all the solutions have a density of 10 gcm3 and a specific heat capacity of 418 jc g calculate the enthalpy change for.
In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hcl are mixed. Assume that no heat is lost to the surroundings or to the calorimeter. Calculate the heat that accompanies this reaction in kjmol of agcl formed.
Both solutions were originally at 246circ mathrmc. After the reaction the final temperature is 3130c. In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hcl are mixed.
Both solutions were originally at 246oc. After the reaction the final temperature is 3130c. After the reaction the final temperature is 3130c.
After the reaction the final temperature is 3138c. In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hcl are mixed. Assuming that all the solutions have a density of 10 gcm 3 and a specific heat capacity of 418 j0cg calculate the enthalpy change for the neutralization of hcl by naoh.
In a coffee cup calorimeter 500 ml of 0100 m agno3 and 500 ml of 0100 m hcl are mixed to yield the following reaction. After the reaction the final temperature is 313circ mathrmc. Solution for in a coffee cup calorimeter 1000 ml of 11 m naoh and 1000 ml of 11 m hcl are mixed.
Assuming that all the solutions have a density of 10 gcm3 and a specific heat capacity of 418 j8c. G calculate the enthalpy change for the neutralization of hcl by naoh. Assuming that all the solutions have a density of 10 gcm 3 and a specific heat capacity of 418 j0cg calculate the enthalpy change for the neutralization of hcl by naoh.
Assuming that all the solutions have a density of 10 mathrmg mathrmcm3 and a specific heat capacity of 418 mathrmj mathrmc cdot mathrmg calculate the enthalpy change for the neutralization.


 Solved 10 In A Coffee Cup Calorimeter 100 0 Ml Of 1 0 M Chegg Com
Solved 10 In A Coffee Cup Calorimeter 100 0 Ml Of 1 0 M Chegg Com

 Solved In A Coffee Cup Calorimeter 100 Ml Of 1 0 M Naoh Chegg Com
Solved In A Coffee Cup Calorimeter 100 Ml Of 1 0 M Naoh Chegg Com
 In A Coffee Cup Calorimeter 100 0 Mathrm Ml Of
In A Coffee Cup Calorimeter 100 0 Mathrm Ml Of
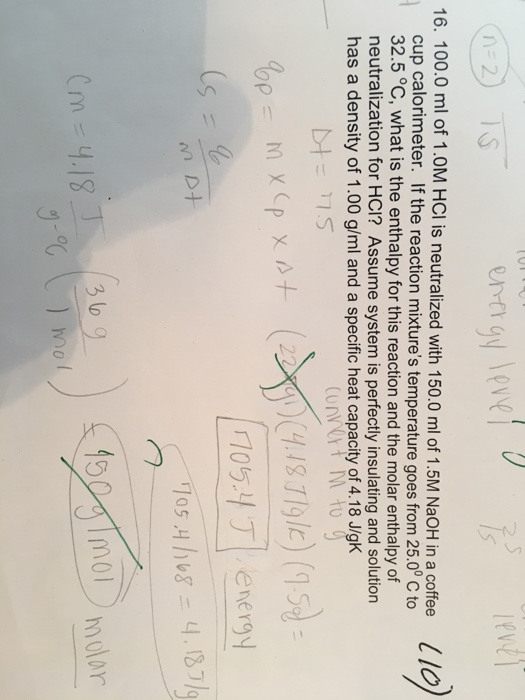
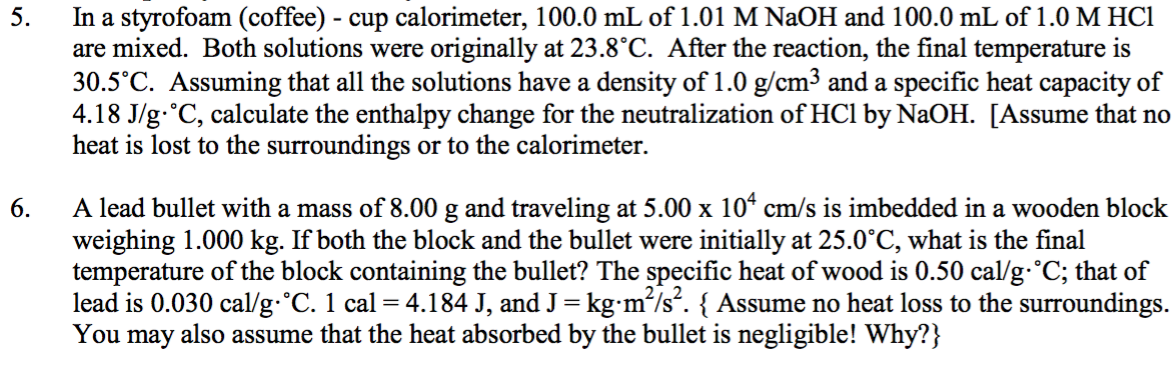 Solved In A Styrofoam Coffee Cup Calorimeter 100 0 M Chegg Com
Solved In A Styrofoam Coffee Cup Calorimeter 100 0 M Chegg Com
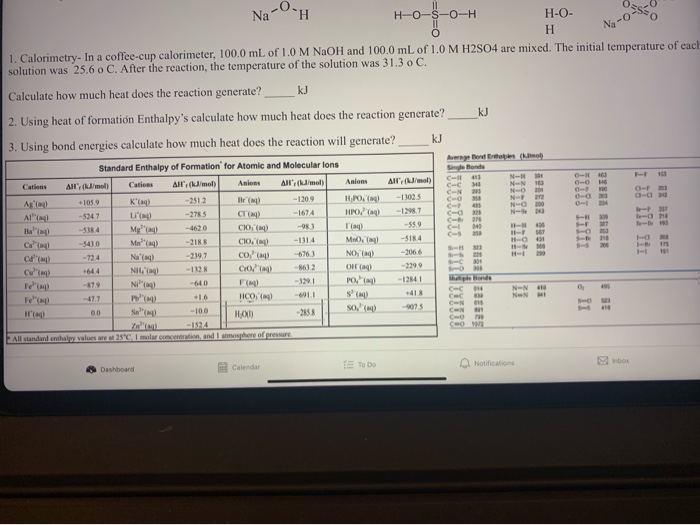 Solved Na Oh H O S O H H 0 0 0 Hnoo 1 Calorimetry In A Chegg Com
Solved Na Oh H O S O H H 0 0 0 Hnoo 1 Calorimetry In A Chegg Com
Http Www Peoriapublicschools Org Cms Lib2 Il01001530 Centricity Domain 2996 Ib 20chem 20 20energetics 20enthalpy 20practice 20problems 20answers Pdf
 Ap Chemistry Ch 6 Thermochemistry Energy Is The Ability To Do Work Or Produce Heat The Sum Of All Of The Potential And Kinetic Energy Ppt Download
Ap Chemistry Ch 6 Thermochemistry Energy Is The Ability To Do Work Or Produce Heat The Sum Of All Of The Potential And Kinetic Energy Ppt Download