A Coffee Cup Calorimeter Contains 250 G Water At 238
Calculate the specific heat of the metal. A coffee cup calorimeter contains 250 g water at 238 c.

a coffee cup calorimeter contains 250 g water at 238
a coffee cup calorimeter contains 250 g water at 238 is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in a coffee cup calorimeter contains 250 g water at 238 content depends on the source site. We hope you do not use it for commercial purposes.
A coffee cup calorimeter contains 1500g of water at 251c.

A coffee cup calorimeter contains 250 g water at 238. A 5 g hot metal at 783 deg. The final temperature of mixture was 463 0c. Delta t t final tinitial.
The final temperature of mixture was 463 degree c. A coffee cup calorimeter contains 250 grams water at 238 degrees c. C is dropped into the.
The specific heat of water is 4184 j g degree c. Of the mixture is 466 deg. Change in teaoe atue a coffee cup calorimeter contains 250 g water at 238 0c.
How do i solve this. Calculate the specific heat of unknown metal if the final temp. The specific heat of cus is 0385 jgk.
The final temperature of mixture was 463 c. The final temperature of mixture 46667 results page 4. Calculate the specific heat of the metal.
A 500 g sample of an unknown metal at an initial temperature of 783 degree c was dropped into the calorimeter. Calculate the specific heat of the metal the specific heat ofwater is 4184 j gc. The final temperature of mixture was 46 3c.
A coffee cup calorimeter contains 250 g water at 238 0c a 500 g sample of an unknown metal at an initial temperature of 7830c was dropped into the calorimeter. The specific heat of water is 4184. A 500 g sample of an unknown metal at an initial temperature of 783 degrees c was dropped into the calorimeter.
A coffee cup calorimeter contains 250 grams water at 238 c a 500g sample of an unknown metal at an initial temperature of 783 c was dropped into the calorimeter. A 500 g sample of an unknown metal at an initial temperature of 783 c was dropped into the calorimeter. Calculate the specific heat of the metal.
A calorimeter contains 25 g of water at 238 degrees c. A coffee cup calorimeter contains 250 grams water at 238 c a 500g sample of an unknown metal at an initial temperature of 783 c was dropped into the calorimeter. The cu is added to the calorimeter and after a time the contents of the cup reach a constant temperature of 301c.
A 1200g block of copper metal is heated to 1004 c by putting it in a beaker of boiling water. The final temperature of mixture was 463 c. The specific heat of ater is 4184 j g c.
A coffee cup calorimeter contains 250 g water at 238 degree c. U know qmcdelta t. A 500 g sample of an unknown metal at an initial temperature of 783 0c was dropped into the calorimeter.
 Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
 Solved Change In Teaoe Atue A Coffee Cup Calorimeter Cont Chegg Com
Solved Change In Teaoe Atue A Coffee Cup Calorimeter Cont Chegg Com
 Solved Change In Teaoe Atue A Coffee Cup Calorimeter Cont Chegg Com
Solved Change In Teaoe Atue A Coffee Cup Calorimeter Cont Chegg Com
 Solved 2 The Heat Of Combustion Of Octane Cgh18 Can Be Chegg Com
Solved 2 The Heat Of Combustion Of Octane Cgh18 Can Be Chegg Com
 Solved E After Completing Lab Answer In Space Provided Chegg Com
Solved E After Completing Lab Answer In Space Provided Chegg Com
 Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
 Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
Oneclass A Coffee Cup Calorimeter Contains 25 0 G Water At 23 8 Degree C A 5 00 G Sample Of An Unkn
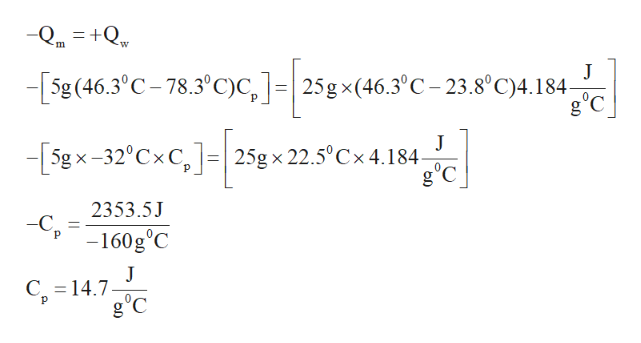 Answered A Coffee Cup Calorimeter Contains 25 0 Bartleby
Answered A Coffee Cup Calorimeter Contains 25 0 Bartleby
 Solved Heat Of Acid Base Neutralization Me Date Post La Chegg Com
Solved Heat Of Acid Base Neutralization Me Date Post La Chegg Com
 Chemistry Thermochemistry Chapter 5 Ppt Download
Chemistry Thermochemistry Chapter 5 Ppt Download
