In The Following Experiment A Coffee Cup Calorimeter Containing
If 620g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. The initial temperature of the calorimeter is 230 c.
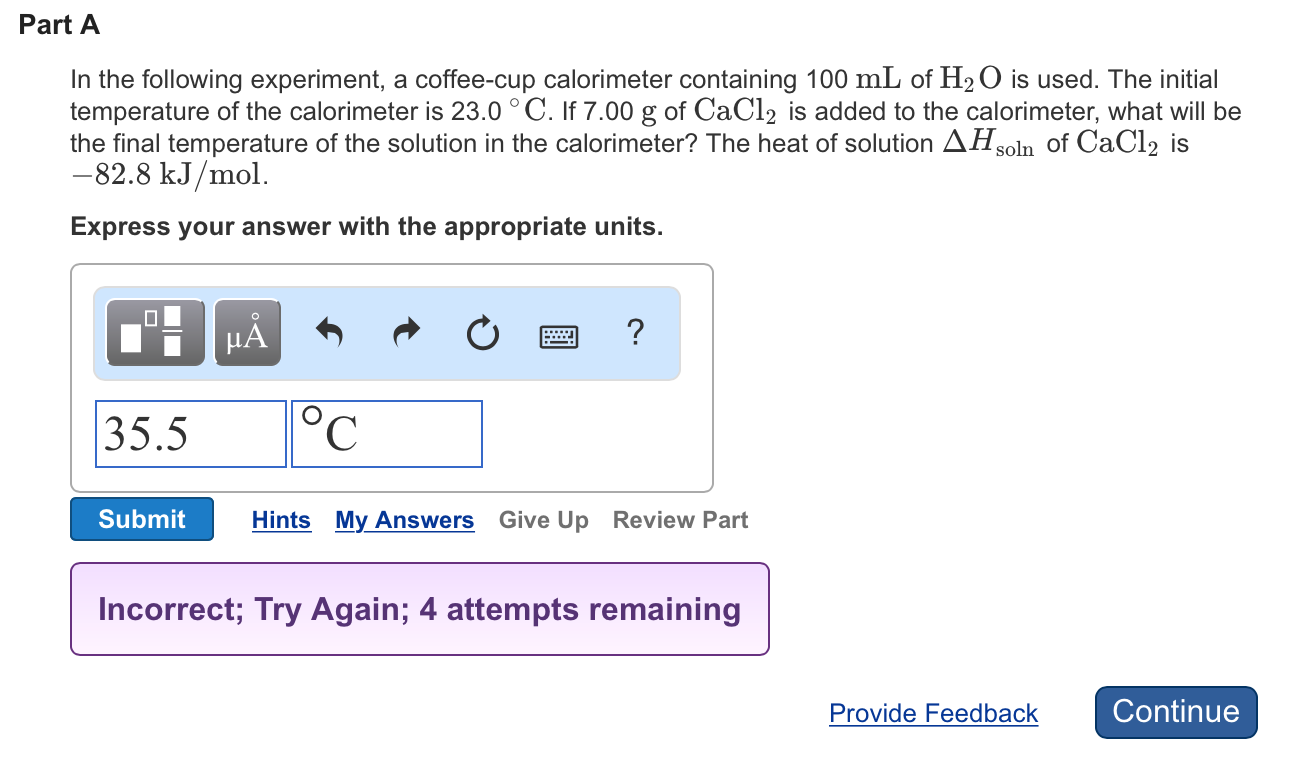 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
in the following experiment a coffee cup calorimeter containing
in the following experiment a coffee cup calorimeter containing is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in in the following experiment a coffee cup calorimeter containing content depends on the source site. We hope you do not use it for commercial purposes.
In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used.

In the following experiment a coffee cup calorimeter containing. In the following experiment a coffee cup calorimeter containing 100 g of h2o is used. In the following experiment a coffee cup calorimeter containing 100. The specific heat of water is cs4184 jgk express your answer.
If 47 g of cacl 2 is added to the calorimeter what will be the final temperature of the solution in the. The initial temperature of the calorimeter is 23c. The heat of the solution is delta h soln of cacl2 is 828 kjmol.
If 900 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. If 970 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. If 730 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter.
The initial temperature of the calorimeter is 230 degrees c. The initial temperature of the calorimeter is 230 c. The initial temperature of the calorimeter is 230 c.
If 800 g of cacl2 is added to the calorimeter what will be the final temperature of the solution in the calorimeter. The initial temperature of the calorimeter is 230 o c. Ml of h2o is used.
The initial temperature of the calorimeter is 230 c. If 250 g of cacl2 is added to the calorimeter what. If 67 g of cacl2 is added to the calorimeter.
The heat of solution dhsolnof cacl2 is 828 kjmol. In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used. In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used.
I got 40 but its incorrect. In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used. Part a in the following experiment a coffee cup calorimeter containing 100 ml of h2o is used.
The heat of solution. The initial temperature of the calorimeter is 230 circ c. In the following experiment a coffee cup calorimeter containing 100 g of h 2 o is used.
The heat of solution dhsoln of cacl2 is 828 kjmol. In the following experiment a coffee cup calorimeter containing 100 ml of h2o is used.
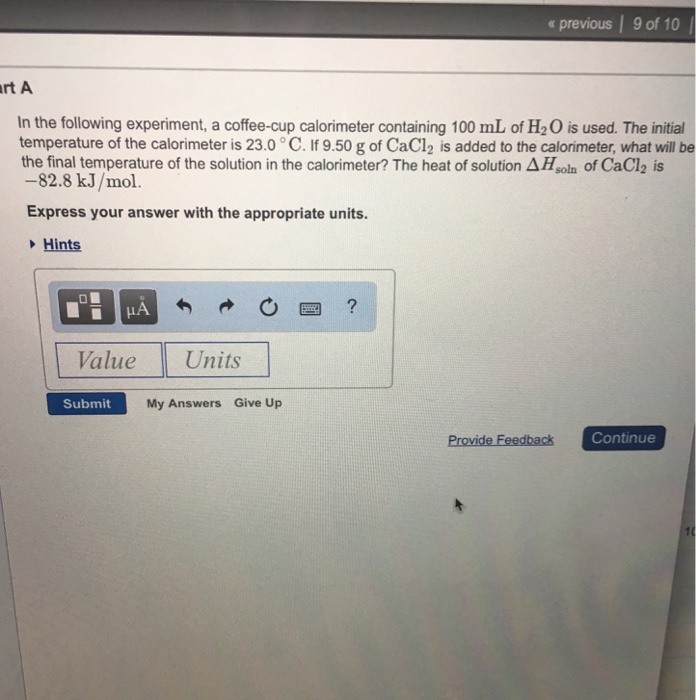
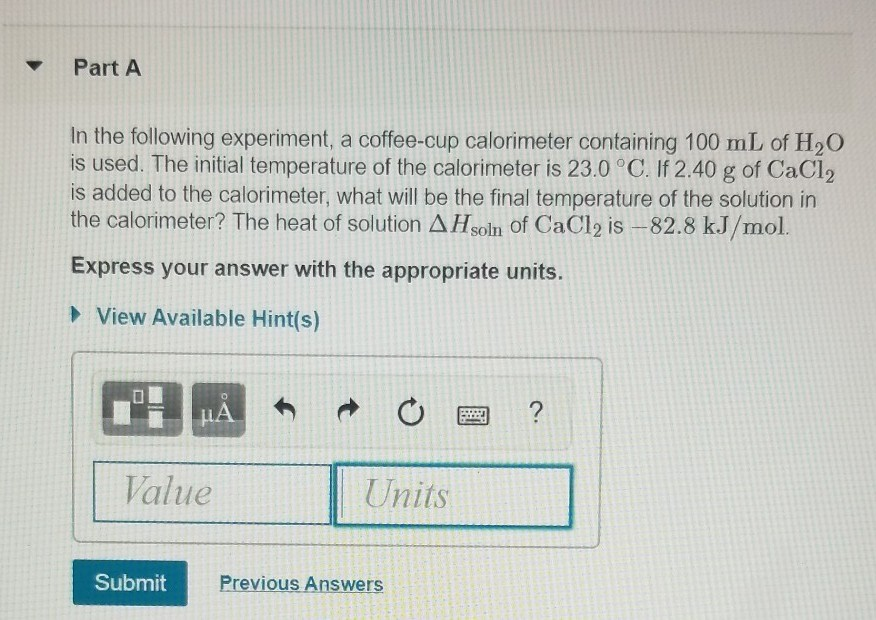 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
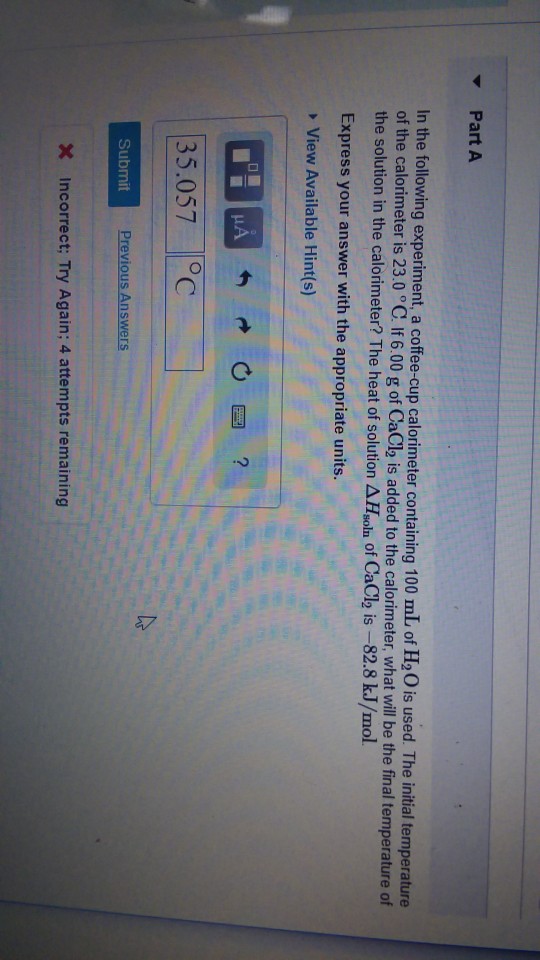
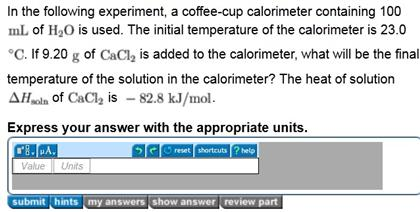 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
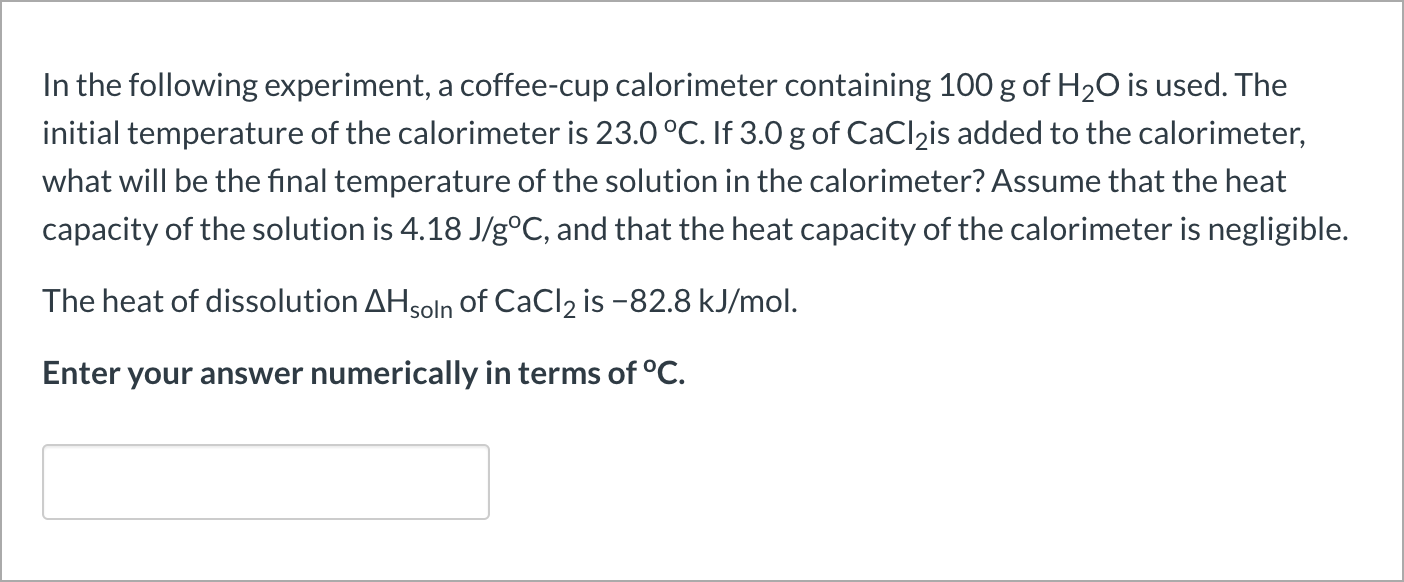 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
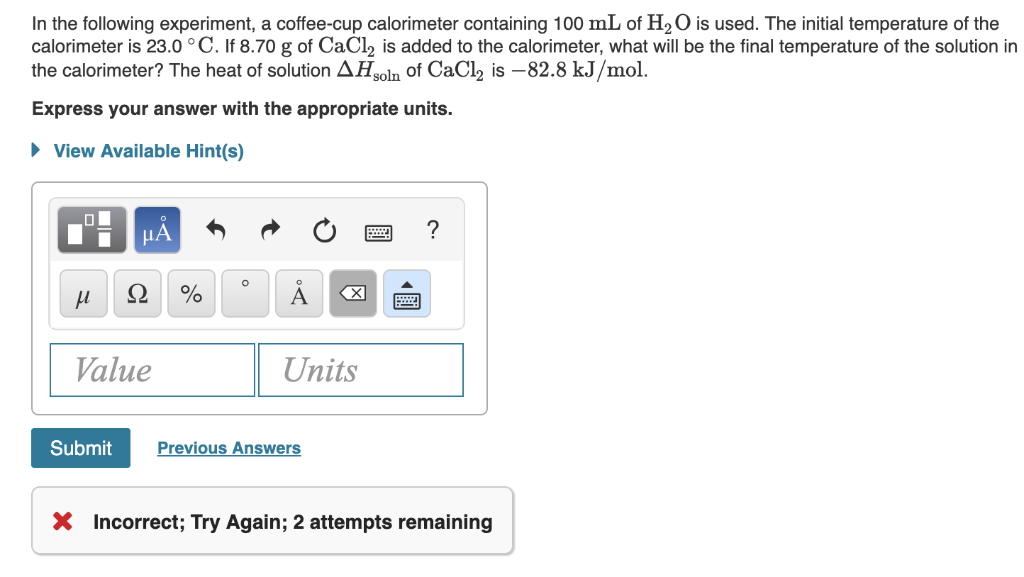 In The Following Experiment A Coffee Cup Calorime Chegg Com
In The Following Experiment A Coffee Cup Calorime Chegg Com
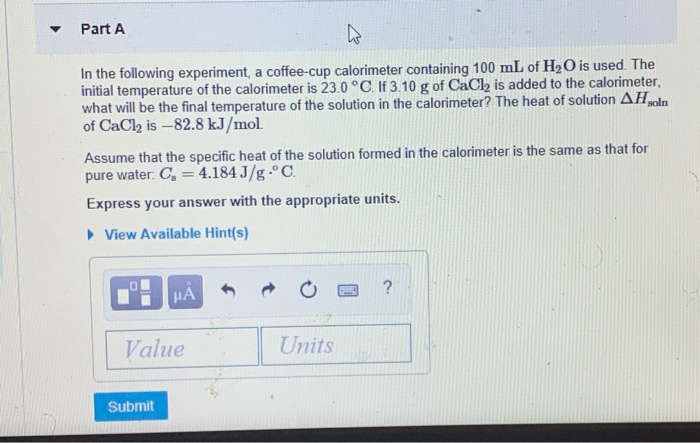 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
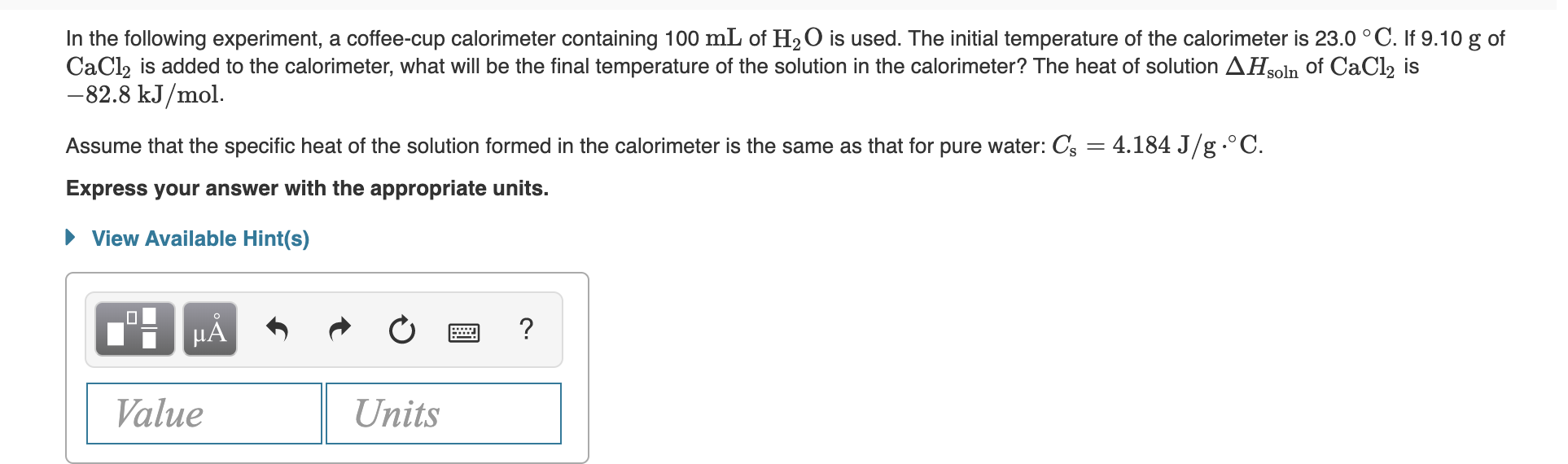 Answered In The Following Experiment A Bartleby
Answered In The Following Experiment A Bartleby
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
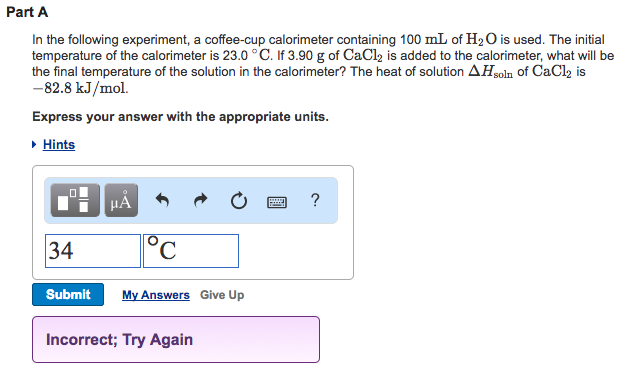 Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
Solved Part A In The Following Experiment A Coffee Cup C Chegg Com
 Solved In The Following Experiment A Coffee Cup Calorime Chegg Com
Solved In The Following Experiment A Coffee Cup Calorime Chegg Com