In A Coffee Cup Calorimeter 1000 Ml Of
After the reaction the final temperature is 313 0c. Given that the density of nacl solution is 1038 gml and the specific heat of nacl solution is 387 jg0c calculate the dhmole for the reaction of hcl with naoh.

in a coffee cup calorimeter 1000 ml of
in a coffee cup calorimeter 1000 ml of is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in in a coffee cup calorimeter 1000 ml of content depends on the source site. We hope you do not use it for commercial purposes.
Ag aq cl aq agcl s the two solutions were initially at.

In a coffee cup calorimeter 1000 ml of. In a coffee cup calorimeter 1000 mathrmml of 10 mathrmm naoh and 1000 mathrmml of 10 mathrmm mathrmhcl are mixed. After the reaction the final temperature is 313circ mathrmc. Assume a mass of 1000.
In a coffee cup calorimeter 500 ml of 0100 m agno3 and 500 ml of 0100 m hcl are mixed to yield the following reaction. Both solutions were originally at 246 0c. In a coffee cup calorimeter 500 ml of 0100 m agno3 and 500 ml of 0100 m hcl are mixed to yield the following reaction.
Both solutions were originally at 246circ mathrmc. After the reaction the final temperature is 3130c. Assuming that all the solutions have a density of 10 mathrmg mathrmcm3 and a specific heat capacity of 418 mathrmj mathrmc cdot mathrmg calculate the enthalpy change for the neutralization.
Assuming that all the solutions have a density of 10 gcm 3 and a specific heat capacity of 418 j0cg calculate the enthalpy change for the neutralization of hcl by naoh. Ag1aq 1 cl2aq 88n agcls if the two solutions are initially at 22608c and if the final temperature is 23408c calculate dh for the reaction in kjmol of agcl formed. Both solutions were originally at 246oc.
Assume that no heat is lost to the surroundings or to the calorimeter. In a coffee cup calorimeter 1000 ml of 10 m naoh and 1000 ml of 10 m hci are mixed. The following reaction occurs.
In a coffee cup calorimeter 500 ml of 100 m naoh and 500 ml of 100 m hcl are mixed. Solution for in a coffee cup calorimeter 1000 ml of 11 m naoh and 1000 ml of 11 m hcl are mixed. Both solutions were originally at 2450c.
In a coffee cup calorimeter 500 ml of 0100 m agno3 and 500 ml of 0100 m hcl are mixed.

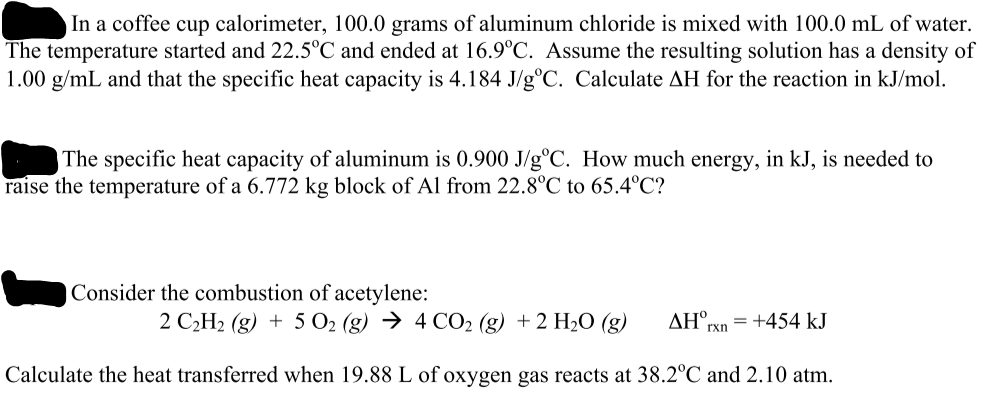 Solved In A Coffee Cup Calorimeter 100 0 Grams Of Alumin Chegg Com
Solved In A Coffee Cup Calorimeter 100 0 Grams Of Alumin Chegg Com

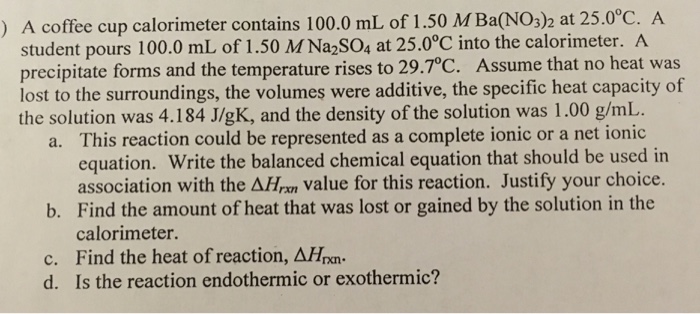
 Solved 6 In A Coffee Cup Calorimeter 100 0 G Of H20 And Chegg Com
Solved 6 In A Coffee Cup Calorimeter 100 0 G Of H20 And Chegg Com
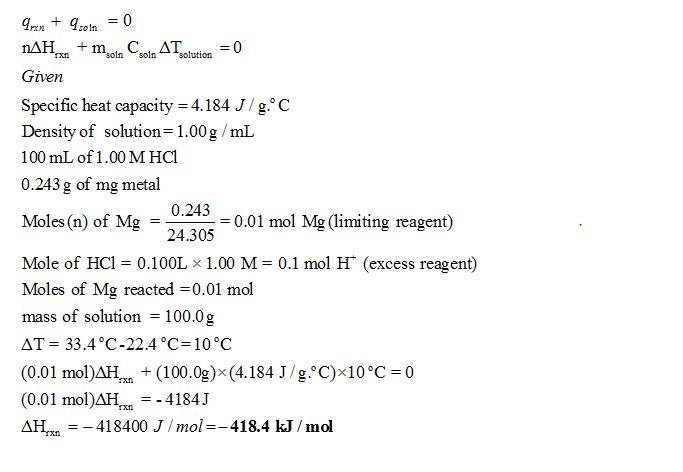 Oneclass A Coffee Cup Calorimeter Contains 100 0 Ml Of 1 00m Hcl At 22 4i C A Sample Of 0 243 G Of
Oneclass A Coffee Cup Calorimeter Contains 100 0 Ml Of 1 00m Hcl At 22 4i C A Sample Of 0 243 G Of
 Solved 10 In A Coffee Cup Calorimeter 100 0 Ml Of 1 0 M Chegg Com
Solved 10 In A Coffee Cup Calorimeter 100 0 Ml Of 1 0 M Chegg Com
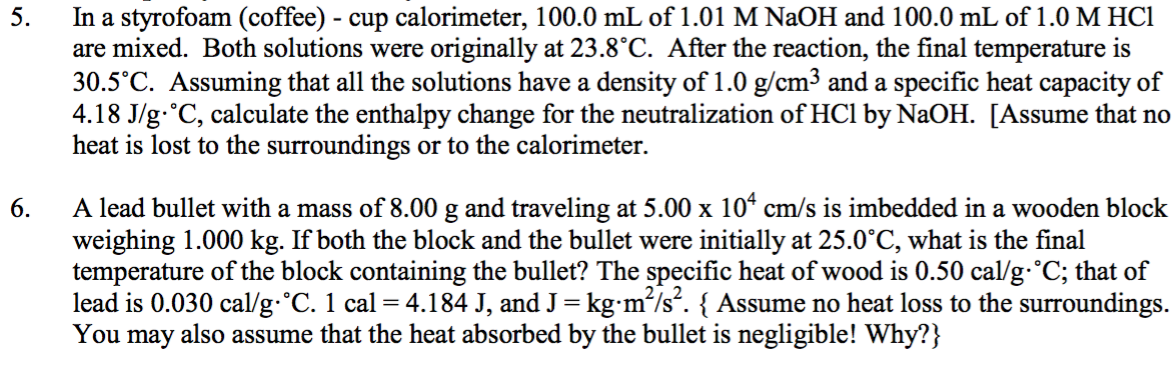 Solved In A Styrofoam Coffee Cup Calorimeter 100 0 M Chegg Com
Solved In A Styrofoam Coffee Cup Calorimeter 100 0 M Chegg Com

 In A Coffee Cup Calorimeter 100 0 Mathrm Ml Of
In A Coffee Cup Calorimeter 100 0 Mathrm Ml Of
